Cannabis and its components have gained increased recognition in public health for their therapeutic potential in recent years. Once criminalized, cannabis has now emerged into a domain where its use and benefits are being explored alongside challenges in regulation and quality control.
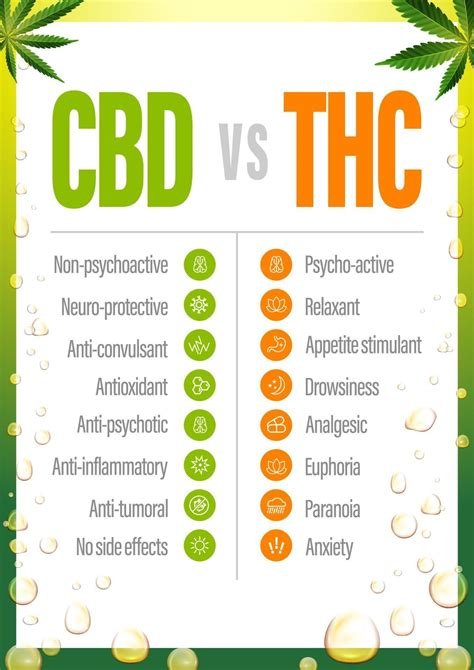
Cannabis consists of more than 100 cannabinoids, with tetrahydrocannabinol (THC) and cannabidiol (CBD) being the most studied. THC is known for its psychoactive effects, while CBD is non-intoxicating and has garnered significant attention for its medical applications. Research indicates that CBD interacts with various receptor systems in the human body, including cannabinoid and serotonin receptors, providing potential therapeutic benefits without the cognitive effects associated with THC.
Studies show that CBD has demonstrated effectiveness in several health areas. The most compelling evidence supports its use in epilepsy treatment, particularly for rare forms such as Dravet syndrome and Lennox-Gastaut syndrome, where CBD has led to a reduction in seizure frequency. The FDA has even approved Epidiolex, a CBD-based medication, for these conditions.
Other health applications of CBD are still being evaluated. Current evidence suggests: – Anxiety disorders: Moderate evidence points to reduced symptoms in clinical trials. – Chronic pain: Moderate studies support CBD’s anti-inflammatory and pain-relieving effects. – Sleep disorders: Preliminary findings indicate improved sleep quality in some individuals. – Addiction management: Early research shows potential in reducing cravings and withdrawal symptoms.
The rise in CBD popularity has influenced agricultural practices, with growers focusing on high-CBD, low-THC strains to meet both consumer demand and regulatory standards. Key agricultural practices include selecting optimal harvest times to enhance CBD content and using organic methods to reduce contaminants. These practices directly impact the quality and safety of CBD products sold to consumers.
Despite the promising findings, public health officials express concerns about product quality and regulation. Unregulated markets have produced discrepancies between labeled and actual cannabinoid content. Reports indicate that many products do not contain the advertised levels of CBD, may have undisclosed THC, and can include harmful contaminants like pesticides or heavy metals. These issues underline the necessity for rigorous quality control standards to ensure consumer safety while maintaining access to beneficial products.
Additionally, there is a gap between preliminary research and the marketing claims made by CBD companies. This disconnect can lead consumers to delay seeking proven medical treatments, experience adverse effects, or invest in products that offer little benefit. Public health professionals warn that exaggerated claims can foster unrealistic expectations about the efficacy of CBD.
Moving forward, a balanced approach to cannabis in public health is essential. It is critical for health professionals, regulatory bodies, and the public to focus on evidence-based practices rather than hype. By addressing the legitimate medical uses of cannabis alongside the pressing need for regulation and quality assurance, public health strategies can better serve diverse populations and maximize the benefits of cannabis.
In summary, while cannabis, particularly CBD, displays considerable therapeutic promise, the landscape remains fraught with challenges that necessitate careful navigation. Establishing robust regulatory frameworks and promoting evidence-based information will be key to unlocking the full potential of cannabis in public health.